Sciences
BackScience has three main disciplines: Biology, Chemistry and Physics. Studying Science helps us understand the natural and physical world around us through experimentation and observation.
Key Stage 3 Science Learning Journey
Biology Curriculum Overview
Key Stage 3
| Year 7 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: |
Life’s Building Blocks |
Organisation in animals |
Life processes - Reproduction |
| New Knowledge: |
State why scientists like to put organisms into groups.
Explain the different ways of grouping organisms.
Evaluate the different ways of grouping organisms.
Describe how to use and label a light microscope to focus on an object
Calculations of magnification
State the 3 different types of cells
Prepare plant and animal slides to view under the microscope
Compare the sizes of cells
Draw a cell
Design a way to model a cell Compare the different ways to model a cell
Label the cell membrane, cytoplasm, mitochondria and nucleus
State the functions of the cell membrane, cytoplasm, mitochondria and nucleus
Describe the functions of the cell membrane, cytoplasm, mitochondria and nucleus
State why we use microscopes in science
Describe how our knowledge has changed over time with advancements of microscopes.
Evaluate the importance of the use of microscopes in science
State the seven life processes
Describe the differences between living and non-living things
Describe and explain the importance of the seven life processes
Give the word equation for respiration
Describe the test for carbon dioxide
Discuss the factors that would cause carbon dioxide levels to increase in the body
State the uses of energy in organisms
Characteristics of life
Cell structure and function
Aerobic and anaerobic respiration
|
Biological organisation within multicellular organisms (with animals as an example organisms) – from cells, to tissues, to organs, to organ systems and finally to a functioning organism
The organ systems found within humans (as an example organism) and within the animal kingdom – how these organ systems and some of the organs work together to allow animals to complete the seven characteristics of life
The cells, tissues and organs making up the skeletal and muscular system in vertebrate organisms
The functions of the skeletal system – support, protection and production of white blood cells
Types of muscle tissue adapted for different functions in humans – skeletal, smooth (intestines and other digestive organs) and cardiac muscle
The interaction between the muscular and skeletal system to allow movement in humans including antagonistic muscle pairs
The process of respiration to release energy required for life processes
|
Structure and function of male and female human reproductive system
Menstrual cycle
Gametes, fertilisation, gestation and birth, to include the effect of maternal lifestyle on the foetus through the placenta
|
| Previous Knowledge Required: |
Characteristics of life
Describe the differences in the life cycles of a mammal, an amphibian, an insect and a bird
Describe the life process of reproduction in some plants and animals.
describe how living things are classified into broad groups according to common
observable characteristics and based on similarities and differences, including microorganisms, plants and animals
give reasons for classifying plants and animals based on specific characteristics.
|
The function of skeletons and muscles in animals including humans
Structure of animal cells (KS3 Life’s building blocks)
Recognise the impact of diet, drugs and lifestyle on the way their bodies function
|
Describe the changes as humans develop to old age.
Draw a timeline to indicate stages in the growth and development of humans.
Learn about the changes experienced in puberty.
Research the gestation periods of other animals and comparing them with humans; by finding out and recording the length and mass of a baby as it grows.
|
| New Skills: |
Use of a microscope.
Preparing a slide.
Use of a Bunsen burner.
Follow a scientific method
Evaluate the hazards and risks of the method above
Draw a bar chart from results
Evaluate a method and suggest improvements
|
Demonstrate an understanding of the functions of organs
Design a table
Demonstrate the idea of muscles working together
Dissect a chicken wing
|
|
| Links to the School Curriculum: |
PE
Citizenship
Maths – presenting data
|
PE
Citizenship
Ethics
|
Citizenship
|
| Independent Activities: |
Make a model plant cell: https://www.bbc.co.uk/bitesize/topics/znyycdm/articles/zrh8jtyMake a model animal cell: https://www.bbc.co.uk/bitesize/topics/znyycdm/articles/z4nj2nb |
Make your own diagram of a human skeleton: |
Research the lifecycle of a non-mammalian organism. |
| Web Links: |
BBC Bitesize https://www.bbc.co.uk/bitesize/subjects/zng4d2pOak National Academy – cells, tissues and organs. Lesson 1,5,7 and 8 |
https://www.bbc.co.uk/bitesize/subjects/zng4d2p |
https://www.bbc.co.uk/bitesize/subjects/zng4d2phttps://classroom.thenational.academy/subjects-by-key-stage/key-stage-3/subjects/science |
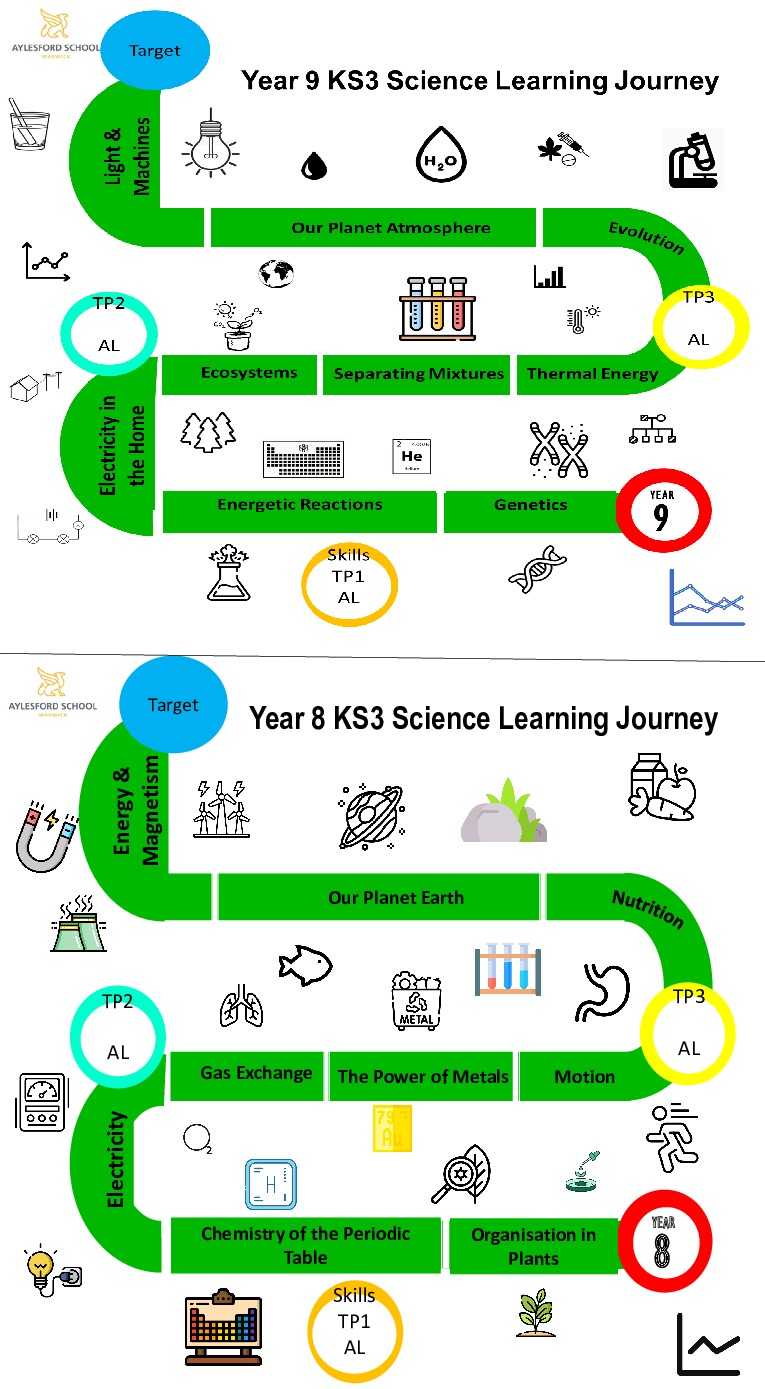
| Year 8 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: |
Organisation in plants |
Reproduction |
Life processes – Nutrition |
| New Knowledge: |
Plant cells, structure and function including the cell wall, permanent vacuole and chloroplasts. Comparison of animal and plant cell structures
Photosynthesis – it’s reactants and products including the general word equation. Storage of glucose as starch, chlorophyll – the green pigment found in plants and sunlight.
Cellular respiration in plants
Plant organs; The leaves as organs of gas exchange and photosynthesis Transport tissues – the phloem and xylem The roots as organs – absorbing water and minerals from the soil. The structure and adaptations of root hair cells.
The uses and importance of soil minerals including nitrogen, potassium and phosphorous
Adaptations of plants in extreme environments (xerophytes) including cacti, water lilies and carnivorous plants The importance of green plants to the human race – would we survive without plants? Role of green plants in life on Earth; production of food crops, foundations of the food chain, release of oxygen, storage of carbon, as fuels, for clothing, medicine, soil stability |
Structure and function of male and female human reproductive system
Menstrual cycle
Gametes, fertilisation, gestation and birth, to include the effect of maternal lifestyle on the foetus through the placenta
|
Meaning of a balanced diet and problems from an unbalanced diet
Digestive system function and adaptation
Importance of bacteria in the digestive system
|
| Previous Knowledge Required: |
identify and describe the functions of different parts of flowering plants: roots, stem/trunk, leaves and flowers
explore the requirements of plants for life and growth (air, light, water, nutrients from soil, and room to grow) and how they vary from plant to plant
investigate the way in which water is transported within plants
explore the part that flowers play in the life cycle of flowering plants, including pollination, seed formation and seed dispersal.
|
Describe the changes as humans develop to old age.
Draw a timeline to indicate stages in the growth and development of humans.
Learn about the changes experienced in puberty.
Research the gestation periods of other animals and comparing them with humans; by finding out and recording the length and mass of a baby as it grows.
|
Identify and name the main parts of the human circulatory system, and describe the functions of the heart, blood vessels and blood recognise the impact of diet, exercise, drugs and lifestyle on the way their bodies function describe the ways in which nutrients and water are transported within animals,
including humans.
|
| New Skills: |
Dissection Performing chromatography |
|
Data analysis Graph drawing skills |
| Links to the School Curriculum: |
Geography KS3 Ecosystems/biomes
|
Citizenship |
Food technology Sport/ P.E |
| Independent Activities: |
Royal Horticultural Society – ideas for starting to grow plants, become involved in community gardening projects. A science tab provides further information on the topics studied in the module as well as linking to future year 9 topics of Ecosystems and biodiversity. The Royal Horticultural society maintains and manages gardens open to the public. BBC iPlayer – The Green Planet – Requires a BBC iPlayer account. Dive into a world where a single life can last a thousand years, with David Attenborough. See things no eye has ever seen, and discover the dramatic, beautiful plant life of Earth |
Research the lifecycle of a non-mammalian organism. | Create a healthy and balanced meal plan for your family for a week |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/znyycdm/articles/zrp3ydmhttps://www.bbc.co.uk/bitesize/topics/zxhhvcw/articles/z6btng8https://www.bbc.co.uk/bitesize/topics/znyycdm/articles/z2d2gdmhttps://www.bbc.co.uk/bitesize/topics/zvrrd2phttps://www.bbc.co.uk/bitesize/topics/zxhhvcw/articles/zvscr2phttps://www.bbc.co.uk/bitesize/topics/zsg6m39 | https://www.bbc.co.uk/bitesize/subjects/zng4d2phttps://classroom.thenational.academy/subjects-by-key-stage/key-stage-3/subjects/science | https://www.bbc.co.uk/bitesize/subjects/zng4d2phttps://classroom.thenational.academy/subjects-by-key-stage/key-stage-3/subjects/sciencehttps://www.youtube.com/c/fuseschool |
| Year 9 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: |
Genetics |
Ecosystems |
Evolution |
| New Knowledge: |
|
Meaning of a balanced diet and problems from an unbalanced diet
Digestive system function and adaptation
Importance of bacteria in the digestive system
|
|
| Previous Knowledge Required: |
|
|
identify how animals and plants are adapted to suit their environment in different ways and that adaptation may lead to evolution. |
| New Skills: |
Extraction of DNA Modelling |
Data analysis Carry out ecological survey techniques |
Create replica fossils
Research skills
Data analysis
Evaluation of evidence
|
| Links to the School Curriculum: |
Citizenship |
Geography KS3 – Ecosystems and human impacts on the environment. Conservation tourism. Biomes. |
Geography KS3 – Ecosystems and human impacts on the environment. Conservation tourism. Tectonic activity/tectonic plates.
|
| Independent Activities: |
https://www.stem.org.uk/resources/collection/3498/dnahttps://www.yourgenome.org/activities/origami-dna/ |
Warwickshire Wildlife Trust – Organisation with information and many free activities in the local area, including nature reserves, looking at wildlife and ecosystems in Warwickshire BBC iPlayer – Climate Change the Facts After one of the hottest years on record, Sir David Attenborough looks at the science of climate change and potential solutions to this global threat. Kew Gardens make your own Terrarium |
Herbert Art Gallery and Museum – Museum in Coventry which houses collections of fossils and extinct organism remains, as well as a wide range of specimens representing extant species and diversity of life on Earth UK Fossil Hunting – Website with information on where to and how to legally collect fossils within the UK BBC iPlayer –Dinosaurs the Final Day with David Attenborough - David Attenborough brings to life, in unprecedented detail, the last days of the dinosaurs. |
| Web Links: | https://www.bbc.co.uk/bitesize/subjects/zng4d2phttps://continuityoak.org.uk/lessons# | https://www.bbc.co.uk/bitesize/topics/zxhhvcwhttps://www.bbc.co.uk/bitesize/topics/zhssgk7https://www.bbc.co.uk/bitesize/topics/zsg6m39 | https://www.bbc.co.uk/bitesize/topics/zpffr82https://www.bbc.co.uk/bitesize/topics/zsg6m39 |
GCSE
| Year 10 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: |
Cell Biology Organisation - Humans |
Disease & Treatment |
Organisation – plants Ecology |
| New Knowledge: |
Cell Biology To explain how the main subcellular structures are related to their functions in animal, plant, bacterial and algal cells. Use a light microscope to observe, draw and label a selection of animal and plant cells.
How microscopy techniques have developed over time and increased our understanding of subcellular structures.
Carry out calculations involving real size, image size and magnification and express answers in standard form.
Use models and analogies to develop explanations of how cells divide (introduction of the cell cycle to be then revisited later on)
Organisation – Humans Develop an understanding of size and scale in relation to cells, tissues, organs and systems.
Explain how the small intestine and lungs in mammals and gills in fish are adapted for exchanging materials.
How substances are transported into and out of cells by diffusion (small intestine/lungs)
How substances are transported into and out of cells by active transport (small intestine)
Relate knowledge of enzymes to Metabolism. Describe the nature of enzyme molecules and relate their activity to temperature and pH changes.
Use the ‘lock and key theory’ as a simplified model to explain enzyme action. Sites of production and the action of amylase, proteases and lipases.
Investigate the effect of pH on the rate of reaction of amylase enzyme.
Structure and functioning of the human heart and lungs, including how lungs are adapted for gaseous exchange.
How the structure of blood vessels relates to their functions.
Know the blood components and their functions. |
The relationship between health and disease and the interactions between different types of disease. The effect of lifestyle factors including diet, alcohol and smoking on the incidence of non-communicable diseases at local, national and global levels. Use qualitative reagents to test for a range of carbohydrates, lipids and proteins. How diseases caused by viruses, bacteria, protists and fungi are spread in animals. Examples of these diseases. The non-specific defence systems of the human body against pathogens and the role of the immune system in the defence against disease. How vaccination will prevent illness and explain the use of antibiotics and other medicines in treating disease. Stem cell treatment. The process of discovery and development of potential new medicines. |
Organisation – plants Explain how the structures of plant tissues are related to their functions. Explain how the structure of root hair cells, xylem and phloem are adapted to their functions.
Ecology Different levels of organisation in an ecosystem from individual organisms to the whole ecosystem The importance of interdependence and competition in a community. Factors for which organisms are competing in a given habitat. Explain how a change in an abiotic or biotic factor would affect a given community given appropriate data or context. Measure the population size of a common species in a habitat. Use sampling techniques to investigate the effect of a factor on the distribution of this species. Explain how organisms are adapted to live in their natural environment Recall that many different materials cycle through the abiotic and biotic components of an ecosystem Explain the importance of the carbon and water cycles to living organisms. Explain the role of microorganisms in cycling materials through an ecosystem by returning carbon to the atmosphere as carbon dioxide and mineral ions to the soil. Explain how waste, deforestation and global warming have an impact on biodiversity Describe both positive and negative human interactions in an ecosystem and explain their impact on biodiversity. |
| Previous Knowledge Required: |
Cell Biology Cells as the fundamental unit of living organisms, including how to observe, interpret and record cell structure using a light microscope The functions of the cell wall, cell membrane, cytoplasm, nucleus, vacuole, mitochondria and chloroplasts The similarities and differences between plant and animal cells
Organisation – Humans The hierarchical organisation of multicellular organisms: from cells to tissues to organs to systems to organisms. The tissues and organs of the human digestive system, including adaptations to function and how the digestive system digests food (enzymes simply as biological catalysts) The importance of bacteria in the human digestive system The structure and functions of the gas exchange system in humans, including adaptations to function The impact of exercise, asthma and smoking on the human gas exchange system Aerobic and anaerobic respiration in living organisms, including the breakdown of organic molecules to enable all the other chemical processes necessary for life A word summary for aerobic respiration The process of anaerobic respiration in humans and micro-organisms, including fermentation, and a word summary for anaerobic respiration The differences between aerobic and anaerobic respiration in terms of the reactants, the products formed and the implications for the organism. |
Disease & Treatment Content of a healthy human diet: carbohydrates, lipids (fats and oils), proteins, vitamins, minerals, dietary fibre and water, and why each is needed |
Organisation – plants The hierarchical organisation of multicellular organisms: from cells to tissues to organs to systems to organisms. Plants making carbohydrates in their leaves by photosynthesis and gaining mineral nutrients and water from the soil via their roots. The role of leaf stomata in gas exchange in plants. The reactants in, and products of, photosynthesis, and a word summary for photosynthesis The dependence of almost all life on Earth on the ability of photosynthetic organisms, such as plants and algae, to use sunlight in photosynthesis to build organic molecules that are an essential energy store and to maintain levels of oxygen and carbon dioxide in the atmosphere The adaptations of leaves for photosynthesis.
Ecology The interdependence of organisms in an ecosystem, including food webs and insect pollinated crops The importance of plant reproduction through insect pollination in human food security How organisms affect, and are affected by, their environment, including the accumulation of toxic materials. The interdependence of organisms in an ecosystem, including food webs and insect pollinated crops The importance of plant reproduction through insect pollination in human food security How organisms affect, and are affected by, their environment, including the accumulation of toxic materials. |
| New Skills: |
Cell Biology Demonstrate an understanding of the scale and size of cells and be able to make order of magnitude calculations, including the use of standard form. Use estimations and explain when they should be used to judge the relative size or area of sub-cellular structures. Explain how the structure of different types of cell relate to their function in a tissue, an organ or organ system, or the whole organism. Explain the importance of cell differentiation. Carry out calculations involving magnification, real size and image size using the formula. Evaluate the practical risks and benefits, as well as social and ethical issues, of the use of stem cells in medical research and treatments. Recognise, draw and interpret diagrams that model diffusion. Recognise, draw and interpret diagrams that model osmosis.
Organisation - Humans Develop an understanding of size and scale in relation to cells, tissues, organs and systems. Describe the nature of enzyme molecules and relate their activity to temperature and pH changes. Carry out rate calculations for chemical reactions. Describe the structure and functioning of the human heart and lungs, including how lungs are adapted for gaseous exchange. Explain how the structure of blood vessels relates to their functions. Recognise different types of blood cells in a photograph or diagram, and explain how they are adapted to their functions. |
Explain how diseases caused by viruses, bacteria, protists and fungi are spread in animals and plants. Explain how the spread of diseases can be reduced or prevented. Describe the non-specific defence systems of the human body against pathogens Explain the role of the immune system in the defence against disease. Explain how vaccination will prevent illness in an individual, and how the spread of pathogens can be reduced by immunising a large proportion of the population. Explain the use of antibiotics and other medicines in treating disease. Describe the process of discovery and development of potential new medicines, including preclinical and clinical testing. Understand that the results of testing and trials are published only after scrutiny by peer review. |
Organisation – plants Explain how the structures of plant tissues are related to their functions. Explain how the structure of root hair cells, xylem and phloem are adapted to their functions. Explain the effect of changing temperature, humidity, air movement and light intensity on the rate of transpiration. Understand and use simple compound measures such as the rate of transpiration.
Ecology Describe different levels of organisation in an ecosystem from individual organisms to the Describe the importance of interdependence and competition in a community. Explain how organisms are adapted to live in their natural environment, given appropriate information. Understand that photosynthetic organisms are the producers of biomass for life on Earth. Interpret graphs used to model predator-prey cycles. Explain how a change in an abiotic factor would affect a given community given appropriate data or context. Explain how a change in a biotic factor might affect a given community given appropriate data or context. Describe how different materials cycle through the abiotic and biotic components of an ecosystem Explain the importance of the carbon and water cycles to living organisms. Explain the role of microorganisms in cycling materials through an ecosystem by returning carbon to the atmosphere as carbon dioxide and mineral ions to the soil. Describe both positive and negative human interactions in an ecosystem and explain their impact on biodiversity.
|
| Links to the School Curriculum: |
Maths - Use prefixes centi, milli, micro and nano +standard form +surface area to volume ratios Ethics – Social and ethical issues PE – Use of isotonic drinks Organisation - Humans D&T – size and scale PE – Enzymes and metabolism, effect of exercise on the body + heart, lungs and blood vessels Maths – Formulae, equations, calculations, units Ethics – Ethics of treatment of blood products |
Maths – Percentage cover PE – Keeping healthy and prevention of disease Food Technology – Importance of sterile techniques in food preparation History – Pandemics/Immunity |
Organisation – plants Maths – Translate information between graphical and numerical form/plot and draw appropriate graphs, selecting appropriate scales for axes History - Extract and interpret information from graphs, charts and tables. Geography – Sampling
Ecology Maths - Extract and interpret information from charts, graphs and tables Geography - Habitats Maths - Extract and interpret information from charts, graphs and tables. Geography – Biodiversity/water and carbon cycles/deforestation History - Explain why evidence is uncertain or incomplete in a complex context. |
| Web Links: |
Cell Biology https://www.bbc.co.uk/bitesize/topics/z2mttv4https://www.freesciencelessons.co.uk/gcse-biology-paper-1/cell-biology/Organisation - Humans https://www.bbc.co.uk/bitesize/topics/zwj22nbhttps://www.bbc.co.uk/bitesize/topics/zgr997hhttps://www.freesciencelessons.co.uk/gcse-biology-paper-1/organisation/https://www.freesciencelessons.co.uk/gcse-biology-paper-1/bioenergetics/ |
https://www.bbc.co.uk/bitesize/topics/z9kww6fhttps://www.freesciencelessons.co.uk/gcse-biology-paper-1/Infection-and-response/ |
Organisation - plants https://www.bbc.co.uk/bitesize/topics/zwj22nbhttps://www.bbc.co.uk/bitesize/topics/zgr997hhttps://www.freesciencelessons.co.uk/gcse-biology-paper-1/organisation/https://www.freesciencelessons.co.uk/gcse-biology-paper-1/bioenergetics/ Ecology part 1 https://www.bbc.co.uk/bitesize/topics/zxxhh39 https://www.bbc.co.uk/bitesize/topics/z2mttv4https://www.freesciencelessons.co.uk/gcse-biology-paper-1/ecology |
| Year 11 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
|
Key Topic: |
Homeostasis Inheritance |
Variation & Evolution |
REVISION |
| New Knowledge: |
Homeostasis Explain how the structure of the nervous system is adapted to its functions. Explain how the various structures in a reflex arc – including the sensory neurone, synapse relay neurone and motor neurone – relate to their function Understand why reflex actions are important. Describe the principles of hormonal coordination and control by the human endocrine system. Identify the position of the following on a diagram of the human body: • pituitary gland • pancreas • thyroid • adrenal gland • ovary • testes. Explain that homeostasis is the regulation of the internal conditions of a cell or organism to maintain optimum conditions for function in response to internal and external changes. Inheritance Use information given to show understanding of the Linnaean system. Describe the impact of developments in biology on classification systems. Describe the structure of DNA and define genome and its importance. Understand that meiosis leads to non-identical cells being formed while mitosis leads to identical cells being formed. Explain how meiosis halves the number of chromosomes in gametes and fertilisation restores the full number of chromosomes. Understand the concept of probability in predicting the results of a single gene cross, but recall that most phenotype features are the result of multiple genes rather than single gene inheritance including sex determination. Some disorders are inherited. These disorders are caused by the inheritance of certain alleles |
Variation & Evolution Explain the impact of selective breeding of food plants and domesticated animals. Describe simply how the genome and its interaction with the environment influence the development of the phenotype of an organism. State that there is usually extensive genetic variation within a population of a species Recall that all variants arise from mutations and that: most have no effect on the phenotype; some influence phenotype; very few determine phenotype. Describe genetic engineering as a process which involves modifying the genome of an organism by introducing a gene from another organism to give a desired characteristic. Explain the potential benefits and risks of genetic engineering in agriculture and in medicine and that some people have objections. Describe evolution as a change in the inherited characteristics of a population over time through a process of natural selection which may result in the formation of a new species. Explain how evolution occurs through natural selection of variants that give rise to phenotypes best suited to their environment. Describe the evidence for evolution including fossils and antibiotic resistance in bacteria. Describe factors which may contribute to the extinction of a species. |
Revision
Application to GCSE questions
Focus on command words
Addressing importance of key vocabulary Using knowledge acquired from required practicals to novel situations. |
| Previous Knowledge Required: |
Homeostasis The function of muscles and examples of antagonistic muscles. Reproduction in humans (as an example of a mammal) and the menstrual cycle (without details of hormones) The tissues and organs of the human digestive system, including adaptations to function and how the digestive system digests food (enzymes simply as biological catalysts) Inheritance Reproduction in humans (as an example of a mammal), including the structure and function of the male and female reproductive systems, menstrual cycle (without details of hormones), gametes, fertilisation, gestation and birth, to include the effect of maternal lifestyle on the foetus through the placenta Heredity as the process by which genetic information is transmitted from one generation to the next A simple model of chromosomes, genes and DNA in heredity, including the part played by Watson, Crick, Wilkins and Franklin in the development of the DNA model. |
Variation & Evolution Differences between species The variation between individuals within a species being continuous or discontinuous, to include measurement and graphical representation of variation The variation between species and between individuals of the same species means some organisms compete more successfully, which can drive natural selection Changes in the environment may leave individuals within a species, and some entire species, less well adapted to compete successfully and reproduce, which in turn may lead to extinction The importance of maintaining biodiversity and the use of gene banks to preserve hereditary material. |
Revision All Biology topics covered in both Year 10 and 11
Cell Biology
Organisation – Humans
Disease and Treatment
Organisation – Plants
Ecology
Homeostasis
Inheritance
Variation and Evolution |
| New Skills: |
Homeostasis Explain that homeostasis is the regulation of the internal conditions of a cell or organism to maintain optimum conditions for function in response to internal and external changes. Explain how the structure of the nervous system is adapted to its functions. Explain how the various structures in a reflex arc – including the sensory neurone, synapse relay neurone and motor neurone – relate to their function. Students should understand why reflex actions are important. Describe the principles of hormonal coordination and control by the human endocrine system. Explain how insulin controls blood glucose (sugar) levels in the body. Compare Type 1 and Type 2 diabetes and explain how they can be treated. Describe the roles of hormones in human reproduction, including the menstrual cycle. Explain the interactions of FSH, oestrogen, LH and progesterone, in the control of the menstrual cycle. Evaluate the different hormonal and non-hormonal methods of contraception. Explain the use of hormones in modern reproductive technologies to treat infertility. Inheritance Maths – Concept of probability Food Technology – Proportions and ratios Ethics – Ethical issues of embryo screening and gene therapy
|
Variation & Evolution Ethics – Theory of evolution and conflict with religion debate/selective breeding ethical issues/genetic engineering PE – human biology History – economic and social factors |
Revision Maths – How marks are applied in mathematical situations and the importance of writing out the whole method to gain every mark. |
| Enrichment Activities: |
Homeostasis Plan and carry out an investigation into the effect of a factor on human reaction time. Identify the position of the following on a diagram of the human body: pituitary gland, pancreas, thyroid, adrenal gland, ovary and testes. Evaluate information around the relationship between obesity and diabetes, and make recommendations taking into account social and ethical issues. Extract information and interpret data from graphs that show the effect of insulin in blood glucose levels in both people with diabetes and people without diabetes. Inheritance Modelling behaviour of chromosomes during meiosis. Discuss the importance of understanding the human genome. Use direct proportion and simple ratios to express the outcome of a genetic cross. Complete a Punnett square diagram and extract and interpret information from genetic crosses and family trees. Construct a genetic cross by Punnett square diagram and use it to make predictions using the theory of probability. |
|
Revision Practicing exam questions linked to specific topics. Looking at mark schemes and examiner reports. Peer marking. Observing required practicals. |
| Links to the School Curriculum: |
Homeostasis PE – Human biological processes & hormones Psychology – The nervous system and thought processes and response, conscious and unconscious part of the brain Geography - Extract and interpret data from graphs, charts and tables Maths - Translate information about reaction times between numerical and graphical forms. Ethics – Ethical and religious issues surrounding contraception & fertility treatment Inheritance Maths – Concept of probability Food Technology – Proportions and ratios Ethics – Ethical issues of embryo screening and gene therapy |
Variation & Evolution Ethics – Theory of evolution and conflict with religion debate & selective breeding ethical issues, genetic engineering PE – human biology History – economic and social factors |
Revision Maths – How marks are applied in mathematical situations and the importance of writing out the whole method to gain every mark. |
| Web Links: |
https://www.bbc.co.uk/bitesize/topics/zwj22nbhttps://www.freesciencelessons.co.uk/gcse-biology-paper-2/homeostasis Inheritance https://www.bbc.co.uk/bitesize/topics/zppffcwhttps://www.freesciencelessons.co.uk/gcse-biology-paper-2/inheritance/ |
https://www.bbc.co.uk/bitesize/topics/zppffcwhttps://www.freesciencelessons.co.uk/gcse-biology-paper-2/variation-and-evolution/ |
Revision https://www.bbc.co.uk/bitesize/subjects/zp266ychttps://www.freesciencelessons.co.uk/gcse-biology-paper-1/https://www.freesciencelessons.co.uk/gcse-biology-paper-2/ecology/ |
Chemistry Curriculum Overview
Key Stage 3
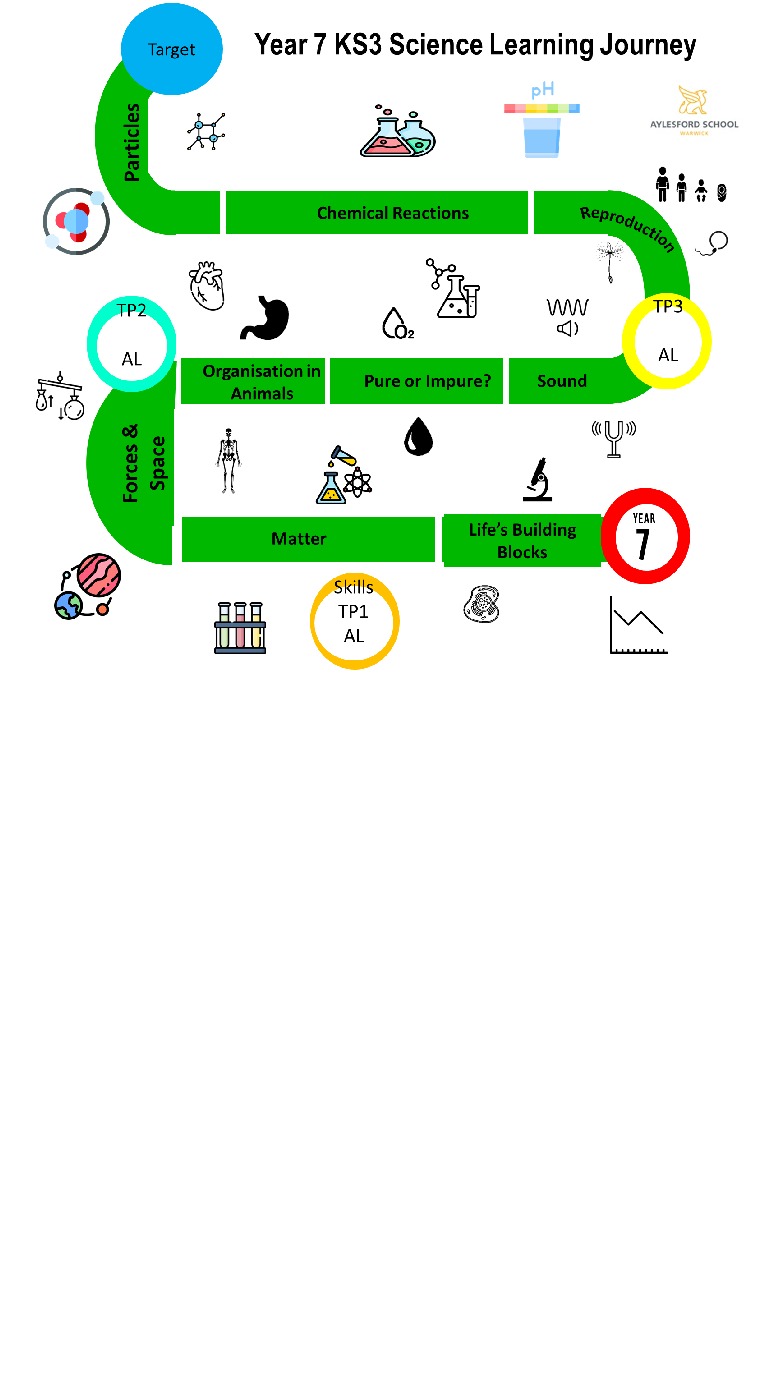
| Year 7 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Matter | Pure or Impure | Chemical Reactions |
| New Knowledge: |
• the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
• a simple (Dalton) atomic model
• differences between atoms, elements and compounds
• chemical symbols and formulae for elements and compounds
• the principles underpinning the Mendeleev Periodic Table
• the physical properties of metals and non-metals
|
• the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
• changes of state in terms of the particle model
• the concept of a pure substance
• mixtures, including dissolving
• the identification of pure substances
|
• Chemical (combustion, oxidation, neutralisation) changes
• physical changes (changes of state)
• pH scale
|
| Previous Knowledge Required: |
• compare and group materials together, according to whether they are solids, liquids or gases.
• observe that some materials change state when they are heated or cooled, and measure or research the temperature at which this happens in degrees Celsius (°C).
• identify the part played by evaporation and condensation in the water cycle and associate the rate of evaporation with temperature.
• compare and group together everyday materials on the basis of their properties, including their hardness, solubility, transparency, conductivity (electrical and thermal), and response to magnets.
• demonstrate that changes of state is a reversible change.
• explain that some changes result in the formation of new materials.
|
• Knowledge of general properties of materials and how a sample could be tested
• Know that some materials are more than one substance that can easily be separated
• know that some materials will dissolve in liquid to form a solution, and describe how to recover a substance from a solution
• give reasons, based on evidence from comparative and fair tests, for the particular uses of everyday materials, including metals, wood and plastic
• demonstrate that dissolving, mixing and changes of state are reversible changes
|
• Reversible and irreversible changes
• Physical Change
|
| New Skills: |
• make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods and suggest possible improvements
• use appropriate techniques, apparatus, and materials during laboratory work, paying attention to health and safety.
|
• ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience
• identifying independent, dependent and control variables
• present observations and data using appropriate methods, including tables and graphs
|
• Constructing word equations
• Modelling scientific laws
• Observations during practicals
• Constructing results tables
• Method writing
|
| Links to the School Curriculum: |
Citizenship Technology |
Citizenship Ethics Technology |
Geography Drama Maths |
| Independent Activities: |
Build an atom simulation: https://edu.rsc.org/resources/iypt-activities-build-an-atom-simulator-activity-sheet/4010253.article |
Use sweets to model atoms, elements, compounds and mixtures: https://stcuthberts.com/media/4958/emc-instagram-challenge.pdf |
Investigating combustion at home: https://www.twinkl.co.uk/resource/us-sc-352-does-fire-need-oxygen-teacher-demonstration-stem-activity-and-resource-pack |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/zstp34jhttps://www.youtube.com/watch?v=14BEh2EKrM0&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=19 | https://www.bbc.co.uk/bitesize/topics/zstp34j/articles/zngddp3?course=zy22qfrhttps://www.youtube.com/watch?v=2i0gv8btYBM&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=15&t=33s | https://www.bbc.co.uk/bitesize/topics/zypsgk7https://www.youtube.com/watch?v=WUSrEKv6x94&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=21 |
| Year 8 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Chemistry of the Periodic Table | Power of Metals | Our Planet - Earth |
| New Knowledge: |
|
|
|
| Previous Knowledge Required: |
|
|
|
| New Skills: |
|
|
|
| Links to the School Curriculum: | Maths | Maths |
Maths Geography |
| Independent Activities: |
Listen to the periodic table song: https://www.youtube.com/watch?v=rz4Dd1I_fX0Choose an element to research and create a poster on it: |
Carry out a survey in your home of where there is something made out of metal. Place your results into a table with the headings; item, where it was found. | Produce a newspaper article on the volcanoes in Iceland and how the volcanoes have impacted on the Icelandic population. |
| Web Links: |
Fuse School: https://www.youtube.com/c/fuseschoolhttps://www.youtube.com/watch?v=lJJCvLIKnSs&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=45&t=2s |
https://www.youtube.com/c/fuseschoolhttps://www.youtube.com/watch?v=ZY3SDgJ5F3Y&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=43&t=85s | https://www.youtube.com/channel/UCDLgcm_hDXh4K99LJsSVHbwhttps://www.youtube.com/watch?v=Kd7-XwBYT2U&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=93https://www.youtube.com/watch?v=WDZOu1fa-tY&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=96 |
| Year 9 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Energetic Reactions | Separating Mixtures | Our Planet - Atmosphere |
| New Knowledge: |
|
|
|
| Previous Knowledge Required: |
|
|
|
| New Skills: |
|
|
|
| Links to the School Curriculum: |
Maths Drama (modelling) |
Maths Food Technology |
Maths Geography History Timelines |
| Independent Activities: | With parental support, you could make a cake carefully weighing out the ingredients before and then the end mass. Is it less, the same or more…why? | With parental support, you could make a cake carefully weighing out the ingredients before and then the end mass. Is it less , the same or more…why? | With parental approval / support, find the best way to separate a yolk from the white. Cook the white is the reaction reversible |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/zypsgk7/articles/zxh7jsghttps://www.bbc.co.uk/bitesize/topics/z9r4jxshttps://www.youtube.com/watch?v=7d5Gsx3-Uio&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=80 | https://www.bbc.co.uk/bitesize/topics/zstp34j/articles/zngddp3https://www.youtube.com/watch?v=WuC3HJkj6uo&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=24&t=3shttps://www.youtube.com/watch?v=dBb0aWXhArk&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=28&t=3s | https://www.bbc.co.uk/bitesize/topics/z3fv4wx/articles/zsgkdp3https://schools.recyclenow.com/wp-content/uploads/2019/12/Home_Recycling_Survey.pdfhttps://www.youtube.com/watch?v=va6p8-7iYKI&list=PLyf3QQ9ddzgngBzZiwWcEBuRoKUYaXS6N&index=97 |
GCSE
| Year 10 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: |
Atomic Structure & Periodic Table. Bonding & Structure. |
Quantitative Chemistry. Chemical Changes. |
Energy Changes. Rate & Extent. |
| New Knowledge: |
Atomic Structure & Periodic Table A simple model of the atom consisting of the nucleus and electrons, relative atomic mass, electronic charge and isotopes
The number of particles in a given mass of a substance
The modern Periodic Table, showing elements arranged in order of atomic number
Position of elements in the Periodic Table in relation to their atomic structure and arrangement of outer electrons
Properties and trends in properties of elements in the same group
Characteristic properties of metals and non-metals
Chemical reactivity of elements in relation to their position in the Periodic Table.
Bonding Changes of state of matter in terms of particle kinetics, energy transfers and the relative strength of chemical bonds and intermolecular forces
Types of chemical bonding: ionic, covalent, and metallic
Bulk properties of materials related to bonding and intermolecular forces
Bonding of carbon leading to the vast array of natural and synthetic organic compounds that occur due to the ability of carbon to form families of similar compounds, chains and rings
Structures, bonding and properties of diamond, graphite, fullerenes and graphene. |
Quantitive Chemistry Calculating relative atomic mass and relative formula mass. Calculating molar quantities (H only). Conservation of mass. Thermal decomposition. Molar ratios (H only). Empirical formula (H only). Calculating excess and limiting reactants (SEP only). Percentage yield and atom economy calculations (SEP only). Calculating gas volumes (SEP only). Concentration of solutions. Titrations (SEP only). Chemical changes Determination of empirical formulae from the ratio of atoms of different kinds Balanced chemical equations, ionic equations and state symbols Identification of common gases The chemistry of acids; reactions with some metals and carbonates pH as a measure of hydrogen ion concentration and its numerical scale Electrolysis of molten ionic liquids and aqueous ionic solutions Reduction and oxidation in terms of loss or gain of oxygen. |
Energy changes Measurement of energy changes in chemical reactions (qualitative) Bond breaking, bond making, and activation energy and reaction profiles (qualitative). Rates of reaction Factors that influence the rate of reaction: varying temperature or concentration, changing the surface area of a solid reactant or by adding a catalyst Factors affecting reversible reactions. Identify catalysts in reactions from their effect on the rate of reaction and because they are not included in the chemical equation for the reaction. The products of the reaction can react to produce the original reactants. Such reactions are called reversible reactions. if a reversible reaction is exothermic in one direction, it is endothermic in the opposite direction. The same amount of energy is transferred in each case. When a reversible reaction occurs in apparatus which prevents the escape of reactants and products, equilibrium is reached when the forward and reverse reactions occur at exactly the same rate. Make qualitative predictions about the effect of changes on systems at equilibrium when given appropriate information (HT ONLY) |
| Previous Knowledge Required: |
Atomic Structure & Periodic Table The properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure Changes of state in terms of the particle model. A simple (Dalton) atomic model Differences between atoms, elements and compounds Chemical symbols and formulae for elements and compounds Simple techniques for separating mixtures: filtration, evaporation, distillation and chromatography Conservation of mass changes of state and chemical reactions. The varying physical and chemical properties of different elements The principles underpinning the Mendeleev Periodic Table The Periodic Table: periods and groups; metals and non-metals How patterns in reactions can be predicted with reference to the Periodic Table The properties of metals and non-metals The chemical properties of metal and non-metal oxides with respect to acidity.
Bonding Differences between atoms, elements and compounds
Chemical symbols and formulae for elements and compounds
Chemical reactions as the rearrangement of atoms
Representing chemical reactions using formulae and using equations |
Quantitive Chemistry Atoms are tiny and mostly empty space. Most of the mass of the atom is in the nucleus. Mass number. Atomic number. Balancing simple equations.
Chemical changes Combustion, thermal decomposition, oxidation and displacement reactions Defining acids and alkalis in terms of neutralisation reactions The pH scale for measuring acidity/alkalinity; and indicators Reactions of acids with metals to produce a salt plus hydrogen Reactions of acids with alkalis to produce a salt plus water What catalysts do. |
Energy changes Exothermic and endothermic chemical reactions (qualitative). Rates of reaction Chemical reactions as the rearrangement of atoms Representing chemical reactions using formulae and using equations Identify catalysts in reactions from their effect on the rate of reaction and because they are not included in the chemical equation for the reaction. Explain catalytic action in terms of activation energy. Make qualitative predictions about the effect of changes on systems at equilibrium when given appropriate information. Interpret appropriate given data to predict the effect of a change in concentration of a reactant or product on given reactions at equilibrium. Interpret appropriate given data to predict the effect of a change in temperature on given reactions at equilibrium. Interpret appropriate given data to predict the effect of pressure changes on given reactions at equilibrium. |
| New Skills: |
Atomic Structure & Periodic Table Calculate the numbers of protons, neutrons and electrons in an atom or ion, given its atomic number and mass number. Represent the electronic structures of the first twenty elements of the periodic table in both forms. Bonding Explain chemical bonding in terms of electrostatic forces and the transfer or sharing of electrons. Work out the charge on the ions of metals and non-metals from the group number of the element, limited to the metals in Groups 1 and 2, and non-metals in Groups 6 and 7. Deduce that a compound is ionic from a diagram of its structure in one of the specified forms Describe the limitations of using dot and cross, ball and stick, two and three-dimensional diagrams to represent a giant ionic structure Work out the empirical formula of an ionic compound from a given model or diagram that shows the ions in the structure. Recognise substances as small molecules, polymers or giant structures from diagrams showing their bonding. Recognise substances as metallic giant structures from diagrams showing their bonding. |
Quantitive Chemistry Using chemical equations to calculate quantities and concentrations. Making up volumetric solutions. Titration technique (SEP only).
Chemical changes Explain how the reactivity of metals with water or dilute acids is related to the tendency of the metal to form its positive ion Deduce an order of reactivity of metals based on experimental results. Interpret or evaluate specific metal extraction processes when given appropriate information Write ionic equations for displacement reactions Identify in a given reaction, symbol equation or half equation which species are oxidised and which are reduced. Explain in terms of gain or loss of electrons, that these are redox reactions Identify which species are oxidised and which are reduced in given chemical equations. Use the formulae of common ions to deduce the formulae of salts. Describe how to make pure, dry samples of named soluble salts from information provided. Explain the terms dilute and concentrated (in terms of amount of substance), and weak and strong (in terms of the degree of ionisation) in relation to acids Predict the products of the electrolysis of binary ionic compounds in the molten state. Predict the products of the electrolysis of aqueous solutions containing a single ionic compound. |
Energy changes Distinguish between exothermic and endothermic reactions on the basis of the temperature change of the surroundings Evaluate uses and applications of exothermic and endothermic reactions given appropriate information. Draw simple reaction profiles (energy level diagrams) for exothermic and endothermic reactions showing the relative energies of reactants and products, the activation energy and the overall energy change, with a curved line to show the energy as the reaction proceeds Use reaction profiles to identify reactions as exothermic or endothermic Explain that the activation energy is the energy needed for a reaction to occur. Calculate the energy transferred in chemical reactions using bond energies supplied. Rates of reaction Draw, and interpret, graphs showing the quantity of product formed or quantity of reactant used up against time Draw tangents to the curves on these graphs and use the slope of the tangent as a measure of the rate of reaction Calculate the gradient of a tangent to the curve on these graphs as a measure of rate of reaction at a specific time. Predict and explain the effects of changes in the size of pieces of a reacting solid in terms of surface area to volume ratio Use simple ideas about proportionality when using collision theory to explain the effect of a factor on the rate of a reaction. Identify catalysts in reactions from their effect on the rate of reaction and because they are not included in the chemical equation for the reaction. Explain catalytic action in terms of activation energy. Make qualitative predictions about the effect of changes on systems at equilibrium when given appropriate information. Interpret appropriate given data to predict the effect of a change in concentration of a reactant or product on given reactions at equilibrium. Interpret appropriate given data to predict the effect of a change in temperature on given reactions at equilibrium. Interpret appropriate given data to predict the effect of pressure changes on given reactions at equilibrium. |
| Links to the School Curriculum: |
Atomic Structure & Periodic Table History – Timelines and historical figures
Maths – Standard form/prefixes/SI units
Geography - Understanding of why and describe how methods and theories develop over time.
Bonding Maths/D&T/Art - Visualise and represent 2D and 3D forms including two-dimensional representations of 3D objects.
Maths/Food Technology - Ratios |
Quantitive Chemistry Maths – calculations and rearranging equations. Physics – principle of conservation of mass.
Chemical changes Geography – Extraction of resources D&T – Materials Maths – Balancing equations/calculations of concentration/converting units |
Energy changes Food Technology – Energy changes Maths – Calculations/negative numbers PE – cold packs as examples of endothermic reactions Rates of reaction Maths - Ratios, fractions and percentages/ Determine the slope and intercept of a linear graph/ Draw and use the slope of a tangent to a curve Geography - Translate information between graphical and numeric form History - Drawing and interpreting appropriate graphs from data PE – Catalysts Geography – Interpreting data Maths – Drawing and interpreting graphs |
| Enrichment Activities: |
Atomic Structure & Periodic Table Describe why the new evidence from the scattering experiment led to a change in the atomic model. Explain the difference between the plum pudding model of the atom and the nuclear model of the atom. Relate size and scale of atoms to objects in the physical world. Bonding Use the idea that intermolecular forces are weak compared with covalent bonds to explain the bulk properties of molecular substances. Visualise and represent 2D and 3D forms including two-dimensional representations of 3D objects. Explain the properties of graphite and diamond in terms of its structure and bonding. Recognise graphene and fullerenes from diagrams and descriptions of their bonding and structure Give examples of the uses of fullerenes, including carbon nanotubes and examples of everyday uses. |
Quantitive Chemistry https://www.bbc.co.uk/bitesize/guides/zs24h39/revision/1 Chemical changes Mixing of reagents to explore chemical changes and/or products. Preparation of a pure, dry sample of a soluble salt from an insoluble oxide or carbonate, using a Bunsen burner to heat dilute acid and a water bath or electric heater to evaporate the solution. Investigate pH changes when a strong acid neutralises a strong alkali. Measure the pH of different acids at different concentrations. Investigate what happens when aqueous solutions are electrolysed using inert electrodes. This should be an investigation involving developing a hypothesis. |
Energy changes Opportunity to measure temperature changes when substances react or dissolve in water. Investigate the variables that affect temperature changes in reacting solutions such as, eg acid plus metals, acid plus carbonates, neutralisations, displacement of metals. Rates of reaction Investigate how changes in concentration affect the rates of reactions by a method involving measuring the volume of a gas produced and a method involving a change in colour or turbidity. Predict and explain using collision theory the effects of changing conditions of concentration, pressure and temperature on the rate of a reaction Investigate the catalytic effect of adding different metal salts to a reaction such as the decomposition of hydrogen peroxide. Opportunities to observe examples of reversible reactions. Use computer simulations to predict and observe dynamic equilibrium. Use computer simulations or video clips to observe the effects of changing conditions of a reversible reaction. |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/zcckk2phttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/atomic-structure-and-the-periodic-table/
Bonding https://www.bbc.co.uk/bitesize/topics/z33rrwxhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/structure-and-bonding/ |
https://www.bbc.co.uk/bitesize/guides/zgcyw6f/revision/1 https://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/structure-and-bonding/ https://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/structure-and-bonding/ |
Energy Changes https://www.bbc.co.uk/bitesize/topics/z27xxfrhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/energy-changes-2/Rates of reaction https://www.bbc.co.uk/bitesize/topics/zwdqqhvhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/rates-of-reaction/ https://www.bbc.co.uk/bitesize/topics/zwdqqhvhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/rates-of-reaction/ |
| Year 11 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic |
Chemical Analysis Organic Chemistry |
Using Resources. Chemistry of the Atmosphere |
Revision
|
| New Knowledge: |
Chemical Analysis Definition of a pure substance. Pure substances have a definite melting point; mixtures melt over a range of temperatures. Examples of formulations and what they are. Explaining how chromatography works and calculating Rf values. Describing tests for common gases. Identifying unknown ions through flame testing and precipitation reactions (SEP only). Instrumental analysis – flame emission spectroscopy (SEP only).
Organic Chemistry A great variety of carbon compounds is possible because carbon atoms can form chains and rings linked by C-C bonds. This branch of chemistry gets its name from the fact that the main sources of organic compounds are living, or once-living materials from plants and animals. These sources include fossil fuels which are a major source of feedstock for the petrochemical industry. Chemists are able to take organic molecules and modify them in many ways to make new and useful materials such as polymers, pharmaceuticals, perfumes and flavourings, dyes and detergents. |
Using Resources Industries use the Earth’s natural resources to manufacture useful products. To operate sustainably, chemists seek to minimise the use of limited resources, use of energy, waste and environmental impact in the manufacture of these products. Chemists also aim to develop ways of disposing of products at the end of their useful life in ways that ensure that materials and stored energy are utilised. Pollution, disposal of waste products and changing land use has a significant effect on the environment, and environmental chemists study how human activity has affected the Earth’s natural cycles, and how damaging effects can be minimised. Chemistry of the atmosphere The Earth’s atmosphere is dynamic and forever changing. The causes of these changes are sometimes man-made and sometimes part of many natural cycles. Scientists use very complex software to predict weather and climate change as there are many variables that can influence this. The problems caused by increased levels of air pollutants require scientists and engineers to develop solutions that help to reduce the impact of human activity |
Revision Application to GCSE questions Focus on command words Addressing importance of key vocabulary Using knowledge acquired from required practicals to novel situations. |
| Previous Knowledge Required: |
Chemical Analysis A pure substance is a single element or compound. Definitions of an atom, element and compound. Chromatography for separating coloured dyes. Common gases can be tested for analytically. Ionic substances are compounds that contain a metal and non-metal component.
Organic Chemistry The properties of the different states of matter (solid, liquid and gas) Chemical symbols and formulae for elements and compounds Simple techniques for separating mixtures: evaporation and distillation Chemical reactions as the rearrangement of atoms Representing chemical reactions using formulae and using equations |
Using Resources The concept of a pure substance and the identification of pure substances. Mixtures, including dissolving. Simple techniques for separating mixtures: filtration, evaporation and distillation Oxidation and displacement reactions The order of metals and carbon in the reactivity series and the use of carbon in obtaining metals from metal oxides Earth as a source of limited resources and the efficacy of recycling
Chemistry of the atmosphere The dependence of almost all life on Earth on the ability of photosynthetic organisms, such as plants and algae, to use sunlight in photosynthesis to maintain levels of oxygen and carbon dioxide in the atmosphere Aerobic respiration in living organisms and how organisms affect, and are affected by changes in their environment. The composition of the atmosphere and the production of carbon dioxide by human activity and the impact on climate. The rock cycle and the formation sedimentary rocks. The carbon cycle |
Revision All Chemistry topics covered in both Year 10 and 11 Atomic Structure Bonding Energy Changes Chemical changes Rates of Reaction Organic Chemistry Chemistry of the atmosphere Using Resources |
| New Skills: |
Chemical Analysis Producing a chromatogram and calculating Rf. Testing for common gases (oxygen, carbon dioxide, chlorine and hydrogen). Carrying out flame tests and precipitation reactions to identify unknown ions (SEP only).
Organic Chemistry Recognise substances as alkanes given their formulae in these forms. Know the names of these specific alkanes - methane, ethane, propane and butane. Explain how fractional distillation works in terms of evaporation and condensation. Write balanced equations for the complete combustion of hydrocarbons with a given formula. Knowledge of trends in properties of hydrocarbons is limited to: boiling points. Viscosity and flammability. Describe in general terms the conditions used for catalytic cracking and steam cracking. Recall the colour change when bromine water reacts with an alkene. Balance chemical equations as examples of cracking given the formulae of the reactants and products. |
Using Resources Distinguish between potable water and pure water Describe the differences in treatment of ground water and salty water Give reasons for the steps used to produce potable water. Comment on the relative ease of obtaining potable water from waste, ground and salt water. Evaluate alternative biological methods of metal extraction Carry out simple comparative LCAs for shopping bags made from plastic and paper.
Chemistry of the atmosphere Interpret evidence and evaluate different theories about the Earth’s early atmosphere. Describe the main changes in the atmosphere over time and some of the likely causes of these changes Describe and explain the formation of deposits of limestone, coal, crude oil and natural gas. Describe the greenhouse effect in terms of the interaction of short and long wavelength radiation with matter. Recall two human activities that increase the amounts of each of the greenhouse gases carbon dioxide and methane. Predict the products of combustion of a fuel given appropriate information about the composition of the fuel and the conditions in which it is used. |
Revision Methods of revision. Unlocking marks. Applying required practicals to novel situations. |
| Links to the School Curriculum: |
Chemical Analysis Maths – calculations. Geography – analysis of impurities in (water) samples. Art – identifying colours in flame tests and precipitation reactions.
Organic Chemistry Geography – Natural resources/fossil fuels/pollution Maths – Balancing equations History – Trends in data |
Using Resources Maths - Translate information between graphical and numeric form. Geography - extract and interpret information about resources from charts, graphs and tables/waste water treatment/sources of water Psychology - Use orders of magnitude to evaluate the significance of data
Chemistry of the atmosphere Maths - Ratios, fractions and percentages. Geography – Earth and the atmosphere/fossil fuels/climate change/global warming History – Interpreting evidence/evaluate Ethics – Models and theories/debates |
Revision Maths – How marks are applied in mathematical situations and the importance of writing out the whole method to gain every mark. |
| Enrichment Activities |
Chemical Analysis https://www.bbc.co.uk/bitesize/guides/zsy4xfr/revision/1 Organic Chemistry Make models of alkane molecules using the molecular modelling kits. Investigate the properties of different hydrocarbons.
Give examples to illustrate the usefulness of cracking. Explain how modern life depends on the uses of hydrocarbons. |
Using Resources State examples of natural products that are supplemented or replaced by agricultural and synthetic products
Distinguish between finite and renewable resources given appropriate information.
Analysis and purification of water samples from different sources, including pH, dissolved solids and distillation.
Chemistry of the atmosphere An opportunity to show that aquatic plants produce oxygen in daylight.
Describe briefly four potential effects of global climate change
Discuss the scale, risk and environmental implications of global climate change.
Describe actions to reduce emissions of carbon dioxide and methane |
Revision Practicing exam questions linked to specific topics. Looking at mark schemes and examiner reports. Peer marking. Observing required practicals. |
| Web Links: |
Chemical Analysis https://www.bbc.co.uk/bitesize/guides/zqqtrwx/revision/1 https://www.bbc.co.uk/bitesize/topics/z9488mnhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/organic-chemistry/ |
https://www.bbc.co.uk/bitesize/topics/zptnng8https://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/resources/ Chemistry of the Atmosphere https://www.bbc.co.uk/bitesize/topics/zysvv9qhttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/the-atmosphere/ |
Revision https://www.bbc.co.uk/bitesize/subjects/zp266ychttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/https://www.freesciencelessons.co.uk/gcse-chemistry-paper-2/ |
Physics Curriculum Overview
Key Stage 3
| Year 7 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Forces. Space | Sound | Particles |
| New Knowledge: |
Forces as pushes or pulls, arising from the interaction between 2 objects. Using force arrows in diagrams, adding forces in 1 dimension, balanced and unbalanced forces. Forces measured in newtons. Non-contact forces: gravity forces acting at a distance on Earth and in space, forces between magnets, and forces due to static electricity. Forces being needed to cause objects to stop or start moving, or to change their speed or direction of motion (qualitative only). Change depending on direction of force and its size.
The seasons and the Earth’s tilt, day length at different times of year, in different hemispheres. Our sun as a star, other stars in our galaxy, other galaxies. The light year as a unit of astronomical distance. |
Frequencies of sound waves, measured in hertz (Hz); echoes, reflection and absorption of sound. Sound needs a medium to travel, the speed of sound in air, in water, in solids. Sound produced by vibrations of objects, in loudspeakers, detected by their effects on microphone. Diaphragm and the ear drum; sound waves are longitudinal. The auditory range of humans and animals. Pressure waves transferring energy; use for cleaning and physiotherapy by ultrasound; waves transferring information for conversion to electrical signals by microphone. |
Conservation of material and of mass, and reversibility, in melting, freezing, evaporation, sublimation, condensation, dissolving. Similarities and differences, including density differences, between solids, liquids and gases. Brownian motion in gases. The difference between chemical and physical changes. Changes with temperature in motion and spacing of particles. Internal energy stored in materials. Diffusion in liquids and gases driven by differences in concentration The difference between chemical and physical changes. Atoms and molecules as particles. |
|
Previous knowledge required: |
Notice that some forces need contact between 2 objects. Explain that unsupported objects fall towards the Earth because of the force of gravity acting between the Earth and the falling object. Identify the effects of air resistance, water resistance and friction, that act between moving surfaces.
Describe the movement of the Earth and other planets relative to the sun in the solar system. Describe the movement of the moon relative to the Earth. Describe the sun, Earth and moon as approximately spherical bodies. Use the idea of the Earth’s rotation to explain day and night and the apparent movement of the sun across the sky. |
Identify how sounds are made, associating some of them with something vibrating. Recognise that vibrations from sounds travel through a medium to the ear Find patterns between the pitch of a sound and features of the object that produced it. Find patterns between the volume of a sound and the strength of the vibrations that produced it. Recognise that sounds get fainter as the distance from the sound source increases |
States of matter Compare and group materials together, according to whether they are solids, liquids or gases. Observe that some materials change state when they are heated or cooled, and measure or research the temperature at which this happens in degrees Celsius (°C). Identify the part played by evaporation and condensation in the water cycle and associate the rate of evaporation with temperature. Properties and changes of materials Use knowledge of solids, liquids and gases to decide how mixtures might be separated, including through filtering, sieving and evaporating. Demonstrate that dissolving, mixing and changes of state are reversible changes. Compare and group together everyday materials on the basis of their properties, including their hardness, solubility, transparency, conductivity (electrical and thermal), and response to magnets. Give reasons, based on evidence from comparative and fair tests, for the particular uses of everyday materials, including metals, wood and plastic |
| New Skills: |
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility. Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience. Make predictions using scientific knowledge and understanding. Apply mathematical concepts and calculate results. Present observations and data using appropriate methods, including tables interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions. Understand and use SI units
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience. Identifying independent, dependent and control variables Make and record observations and measurements. Present observations and data using appropriate methods, including tables and graphs. Make predictions using scientific knowledge and understanding. Use appropriate techniques, apparatus, and materials during laboratory work, paying attention to health and safety. Apply mathematical concepts and calculate results. |
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility. Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience. Make predictions using scientific knowledge and understanding Apply mathematical concepts and calculate results. Present observations and data using appropriate methods, including tables. Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions. Understand and use SI units. Use and derive simple equations and carry out appropriate calculations. |
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility. Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience. Make predictions using scientific knowledge and understanding Apply mathematical concepts and calculate results. Present observations and data using appropriate methods, including tables. Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions. Understand and use SI units. Use and derive simple equations and carry out appropriate calculations |
| Links to the School Curriculum: |
Maths calculations. Design and technology – forces, drag. |
Maths calculations. Music - pitch |
Maths calculation. Design and Technology. Food technology. |
| Independent Activities: |
https://www.bbc.co.uk/bitesize/articles/zv4jdp3#z2d2qfrVisit The National Space Centre in Leicester. |
|
|
| Web Links: | https://www.bbc.co.uk/bitesize/articles/zs3896fhttps://www.bbc.co.uk/bitesize/topics/z8c9q6fhttps://www.bbc.co.uk/bitesize/articles/z7jvf82 | https://www.bbc.co.uk/bitesize/articles/zpm3r2phttps://www.bbc.co.uk/bitesize/topics/zvsf8p3/articles/zh28jsg#zd4vg7 | https://www.bbc.co.uk/bitesize/topics/z9r4jxs |
| Year 8 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key topic: | Electricity. | Motion. | Energy. Magnetism. |
| New Knowledge: |
Electric current is measured in amperes Differences between series and parallel circuits Current is a flow of charge Potential difference, measured in volts, battery and bulb ratings; resistance, measured in ohms, as the ratio of potential difference (p.d.) to current Differences in resistance between conducting and insulating components (quantitative) |
Speed and the quantitative relationship between average speed, distance and time (speed = distance ÷ time). The representation of a journey on a distance-time graph. Relative motion: trains and cars passing one another. |
Energy:
|
| Previous Knowledge Required: |
Identify common appliances that run on electricity Construct a simple series electrical circuit, identifying and naming its basic parts, including cells, wires, bulbs, switches and buzzers Identify whether or not a lamp will light in a simple series circuit, based on whether or not the lamp is part of a complete loop with a battery Associate the brightness of a lamp or the volume of a buzzer with the number and voltage of cells used in the circuit Compare and give reasons for variations in how components function, including the brightness of bulbs, the loudness of buzzers and the on/off position of switches Use recognised symbols when representing a simple circuit in a diagram |
KS2 Mathematics speed could be introduced. |
Energy: Living things and their habitats describe how animals obtain their food from plants and other animals, using the idea of a simple food chain, and identify and name different sources of food. Animals, including humans find out about and describe the basic needs of animals, including humans, for survival (water, food and air). Describe the importance for humans of exercise, eating the right amounts of different types of food, and hygiene.
Magnetism: Observe how magnets attract or repel each other and attract some materials and not others. Compare and group together a variety of everyday materials on the basis of whether they are attracted to a magnet, and identify some magnetic materials. Describe magnets as having 2 poles predict whether 2 magnets will attract or repel each other, depending on which poles are facing. |
| New Skills: |
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience Identifying independent, dependent and control variables Use appropriate techniques, apparatus, and materials during laboratory work, paying attention to health and safety Make and record observations and measurements Present observations and data using appropriate methods, including tables and graphs |
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience.
|
Energy: Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods and suggest possible improvements.
|
| Links to the School Curriculum: |
Maths Childcare |
Maths PE |
Food Technology Maths Geography |
| Independent Activities: | Looking at energy values that different foods have. |
https://www.bbc.co.uk/bitesize/topics/zrvbkqt/articles/zfb6pbk |
|
| Web Links: | https://www.bbc.co.uk/bitesize/topics/zgy39j6/articles/z8mxgdm | https://www.bbc.co.uk/bitesize/topics/z4brd2p/articles/zw9qwnbhttps://www.bbc.co.uk/teach/class-clips-video/articles/zbjs3dm | https://www.bbc.co.uk/bitesize/topics/zc3g87hhttps://www.bbc.co.uk/bitesize/topics/zrvbkqt |
| Year 9 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Electricity in the Home. | Thermal Energy. | Light. Machines. |
| New Knowledge: |
Fuels and energy resources Non-contact forces: gravity forces acting at a distance on Earth and in space, forces between magnets, and forces due to static electricity The magnetic effect of a current, electromagnets, DC motors (principles only) Separation of positive or negative charges when objects are rubbed together: transfer of electrons, forces between charged objects The idea of electric field, forces acting across the space between objects not in contact |
Heating and thermal equilibrium: temperature difference between 2 objects leading to energy transfer from the hotter to the cooler one, through contact (conduction) or radiation; such transfers tending to reduce the temperature difference; use of insulators Comparing the starting with the final conditions of a system and describing increases and decreases in the amounts of energy associated with temperatures |
Fuels and energy resources Non-contact forces: gravity forces acting at a distance on Earth and in space, forces between magnets, and forces due to static electricity The magnetic effect of a current, electromagnets, DC motors (principles only) Separation of positive or negative charges when objects are rubbed together: transfer of electrons, forces between charged objects The idea of electric field, forces acting across the space between objects not in contact Heating and thermal equilibrium: temperature difference between 2 objects leading to energy transfer from the hotter to the cooler one, through contact (conduction) or radiation; such transfers tending to reduce the temperature difference; use of insulators Comparing the starting with the final conditions of a system and describing increases and decreases in the amounts of energy associated with temperatures The similarities and differences between light waves and waves in matter Light waves travelling through a vacuum; speed of light The transmission of light through materials: absorption, diffuse scattering and specular reflection at a surface Use of ray model to explain imaging in mirrors, the pinhole camera, the refraction of light and action of convex lens in focusing (qualitative); the human eye Light transferring energy from source to absorber, leading to chemical and electrical effects; photosensitive material in the retina and in cameras Colours and the different frequencies of light, white light and prisms (qualitative only); differential colour effects in absorption and diffuse reflection Simple machines give bigger force but at the expense of smaller movement (and vice versa): product of force and displacement unchanged |
|
Previous knowledge required: |
Recognise some common conductors and insulators, and associate metals with being good conductors Identify common appliances that run on electricity |
Observe that some materials change state when they are heated or cooled, and measure or research the temperature at which this happens in degrees Celsius (°C) |
Recognise some common conductors and insulators, and associate metals with being good conductors Identify common appliances that run on electricity · observe that some materials change state when they are heated or cooled, and measure or research the temperature at which this happens in degrees Celsius (°C) Recognise that they need light in order to see things and that dark is the absence of light Notice that light is reflected from surfaces Recognise that light from the sun can be dangerous and that there are ways to protect their eyes Recognise that shadows are formed when the light from a light source is blocked by an opaque object Find patterns in the way that the size of shadows change Recognise that light appears to travel in straight lines Use the idea that light travels in straight lines to explain that objects are seen because they give out or reflect light into the eye Explain that we see things because light travels from light sources to our eyes or from light sources to objects and then to our eyes Use the idea that light travels in straight lines to explain why shadows have the same shape as the objects that cast them Recognise that some mechanisms including levers, pulleys and gears allow a smaller force to have a greater effect
|
| New Skills: |
Make predictions using scientific knowledge and understanding Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables Use appropriate techniques, apparatus, and materials during laboratory work, paying attention to health and safety Make and record observations Present observations and data using appropriate methods, including tables and graphs Apply mathematical concepts and calculate results |
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility Make predictions using scientific knowledge and understanding Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods and suggest possible improvements Apply sampling techniques Present observations and data using appropriate methods, including tables and graphs Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions Present reasoned explanations, including explaining data in relation to predictions and hypotheses Understand and use SI units and IUPAC (International Union of Pure and Applied Chemistry) chemical nomenclature |
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience Make predictions using scientific knowledge and understanding Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods and suggest possible improvements Apply mathematical concepts and calculate results Present observations and data using appropriate methods, including tables and graphs Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions Apply mathematical concepts and calculate results Use and derive simple equations and carry out appropriate calculations
|
| Links to the school curriculum: | Geography – types of energy resources and energy conservation Maths – calculations Technology – household electrical appliances; investigating materials that are conductors and insulators |
Technology – heat transfer of materials (e.g. for building and cooking) Maths - calculations |
Art – use of light, colour and shadow Technology – how simple machines work Maths - calculations |
| Independent activities: | https://www.youtube.com/watch?v=2p3dr2Oyids | https://www.thenational.academy/teachers/programmes/science-secondary-ks3/units/hidden-forces/lessons | |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/zgy39j6/articles/zd794xs#zw2k96fhttps://www.bbc.co.uk/bitesize/topics/zgy39j6/articles/z2s6cj6 | https://www.bbc.co.uk/bitesize/topics/zc3g87h/articles/znw7jsg |
https://www.bbc.co.uk/bitesize/topics/zw982hv/articles/z7rckty https://www.bbc.co.uk/bitesize/topics/z4brd2p/articles/z96g3j6 |
GCSE
| Year 10 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Particle model of matter & Atomic structure | Energy & Electricity | Magnetism & Electromagnetism |
| New Knowledge: |
Particle model of matter Relating models of arrangements and motions of the molecules in solid, liquid and gas phases to their densities Melting, evaporation, and sublimation as reversible changes Calculating energy changes involved on heating, using specific heat capacity; and those involved in changes of state, using specific latent heat Links between pressure and temperature of a gas at constant volume, related to the motion of its particles (qualitative). Atomic structure The nuclear model and its development in the light of changing evidence Masses and sizes of nuclei, atoms and small molecules Differences in numbers of protons, and neutrons related to masses and identities of nuclei, isotope characteristics and equations to represent changes Ionisation; absorption or emission of radiation related to changes in electron orbits Radioactive nuclei: emission of alpha or beta particles, neutrons, or gamma-rays, related to changes in the nuclear mass and/or charge Radioactive materials, half-life, irradiation, contamination and their associated hazardous effects, waste disposal Nuclear fission, nuclear fusion and our sun’s energy |
Energy Energy changes in a system involving heating, doing work using forces, or doing work using an electric current: calculating the stored energies and energy changes involved Power as the rate of transfer of energy Conservation of energy in a closed system, dissipation Calculating energy efficiency for any energy transfers Renewable and non-renewable energy sources used on Earth, changes in how these are used
Electricity Measuring resistance using p.d. and current measurements Exploring current, resistance and voltage relationships for different circuit elements; including their graphical representations Quantity of charge flowing as the product of current and time Drawing circuit diagrams; exploring equivalent resistance for resistors in series The domestic a.c. supply; live, neutral and earth mains wires, safety measures Power transfer related to p.d. and current, or current and resistance |
Magnetism & Electromagnetism Exploring the magnetic fields of permanent and induced magnets, and the Earth’s magnetic field, using a compass Magnetic effects of currents, how solenoids enhance the effect How transformers are used in the national grid and the reasons for their use |
| Previous Knowledge Required: |
Particle model of matter Conservation of material and of mass, and reversibility, in melting, freezing, evaporation, sublimation, condensation, dissolving Similarities and differences, including density differences, between solids, liquids and gases Brownian motion in gases Diffusion in liquids and gases driven by differences in concentration The difference between chemical and physical changes. The differences in arrangements, in motion and in closeness of particles explaining changes of state, shape and density, the anomaly of ice-water transition Atoms and molecules as particles. Changes with temperature in motion and spacing of particles Internal energy stored in materials.
Atomic structure A simple (Dalton) atomic model Atoms and molecules as particles |
Energy Comparing power ratings of appliances in watts (W, kW) Comparing amounts of energy transferred (J, kJ, kW hour) Fuels and energy resources Heating and thermal equilibrium: temperature difference between 2 objects leading to energy transfer from the hotter to the cooler one, through contact (conduction) or radiation; such transfers tending to reduce the temperature difference; use of insulators Other processes that involve energy transfer: changing motion, dropping an object, completing an electrical circuit, stretching a spring, metabolism of food, burning fuels Comparing the starting with the final conditions of a system and describing increases and decreases in the amounts of energy associated with movements, temperatures, changes in positions in a field, in elastic distortions and in chemical compositions
Electricity Electric current, measured in amperes, in circuits, series and parallel circuits, currents add where branches meet and current as flow of charge Potential difference, measured in volts, battery and bulb ratings; resistance, measured in ohms, as the ratio of potential difference (p.d.) to current Differences in resistance between conducting and insulating components (quantitative) Separation of positive or negative charges when objects are rubbed together: transfer of electrons, forces between charged objects The idea of electric field, forces acting across the space between objects not in contact |
Magnetism & Electromagnetism Separation of positive or negative charges when objects are rubbed together: transfer of electrons, forces between charged objects The idea of electric field, forces acting across the space between objects not in contact Magnetic poles, attraction and repulsion Magnetic fields by plotting with compass, representation by field lines Earth’s magnetism, compass and navigation The magnetic effect of a current, electromagnets, DC motors (principles only) |
| New Skills: |
Particle model of matter Recall and apply this equation to changes where mass is conserved. Calculate the energy change involved when the temperature of a material changes. Calculate the energy change involved in a change of state. Atomic structure Recognise expressions given in standard form. The ways in which scientific methods and theories develop over time Using a variety of concepts and models to develop scientific explanations and understanding Using scientific theories and explanations to develop hypotheses |
Energy The link between work done (energy transfer) and current flow in a circuit. Recall and apply equations for the kinetic energy of a moving object, the amount of elastic potential energy stored in a stretched spring, the amount of gravitational potential energy gained by an object raised above ground level, the amount of energy stored in or released from a system as its temperature changes and the energy efficiency for any energy transfer. Investigate thermal conductivity using rods of different materials. Electricity Recall and apply the equations for: The size of the electric current is the rate of flow of electrical charge/ calculating current, potential difference or resistance/ how the power transfer in any circuit device is related to the potential difference across it and the current through it, and to the energy changes over time/ The amount of energy transferred by electrical work Translating data from one form to another Using prefixes and powers of ten for orders of magnitude (e.g. tera, giga, mega, kilo, centi, milli, micro and nano) Interconverting units Using an appropriate number of significant figures in calculations |
Magnetism & Electromagnetism Apply the equation for a conductor at right angles to a magnetic field and carrying a current. Describe how to plot the magnetic field pattern of a magnet using a compass Draw the magnetic field pattern of a bar magnet showing how strength and direction change from one point to another Explain how the behaviour of a magnetic compass is related to evidence that the core of the Earth must be magnetic. |
| Links to the School Curriculum: |
Particle model of matter Geography – Tectonics – convection currents Maths – Apply and manipulate equations Atomic structure Maths – Using standard form History – Historical figures |
Energy Maths – Apply and manipulate equations Maths - Calculate or use efficiency values as a decimal or as a percentage D&T – Sources of energy Electricity Maths – Apply and manipulate equations Geography – Translating data from one form to another Food technology – using prefixes/converting units D&T – Electricity D&T – Variables & measurements |
Magnetism & Electromagnetism Psychology - Using scientific theories and explanations to develop hypotheses Evaluating methods and suggesting possible improvements and further investigations Drama/Music – Practical skills, following instructions |
| Enrichment Activities: |
Particle model of matter Perform an experiment to measure the latent heat of fusion of water Atomic structure Read around the scientific theories surrounding atomic structure. Research the involvement of other scientists not listed on the GCSE syllabus to give a larger overview of the history of atomic structure. |
Energy Give examples that illustrate the definition of power eg comparing two electric motors that both lift the same weight through the same height but one does it faster than the other. Describe, with examples, how in all system changes energy is dissipated, so that it is stored in less useful ways. This energy is often described as being ‘wasted’. Electricity Investigate the relationship between the resistance of a thermistor and temperature. Investigate the relationship between the resistance of an LDR and light intensity. Use circuit diagrams to set up and check appropriate circuits to investigate the factors affecting the resistance of electrical circuits. This should include:
|
Magnetism & Electromagnetism Describe the attraction and repulsion between unlike and like poles for permanent magnets. Describe the difference between permanent and induced magnets. Research the uses of magnets and electromagnets in everyday life. Find examples of magnets and electromagnets in your own home. |
| Web Links: |
Particle model of matter: https://www.bbc.co.uk/bitesize/topics/z3ybb82https://www.freesciencelessons.co.uk/gcse-physics-paper-1/particle-model-of-matter/Atomic structure https://www.bbc.co.uk/bitesize/topics/zcckk2phttps://www.freesciencelessons.co.uk/gcse-chemistry-paper-1/atomic-structure-and-the-periodic-table/ |
Energy: https://www.bbc.co.uk/bitesize/topics/z89ddxshttps://www.freesciencelessons.co.uk/gcse-physics-paper-1/energy/Electricity https://www.bbc.co.uk/bitesize/topics/zcg44qthttps://www.freesciencelessons.co.uk/gcse-physics-paper-1/electricity/ |
Magnetism & Electromagnetism: https://www.bbc.co.uk/bitesize/topics/zwkww6fhttps://www.freesciencelessons.co.uk/gcse-physics-paper-2/magnetism/ |
| Year 11 | Term 1 | Term 2 | Term 3 |
|---|---|---|---|
| Key Topic: | Forces |
Waves Space (separates only) |
Revision |
| New Knowledge: |
Describing motion along a line Speed and velocity Acceleration Newton’s laws of motion Forces and braking Momentum (HT only) |
Scalar and vector quantities Contact and non-contact forces Resultant forces Work done and energy transfer Forces and elasticity |
Waves in air, fluids and solids Transverse and longitudinal waves Properties of waves Electromagnetic waves and their properties |
| Previous Knowledge Required: |
Speed and the quantitative relationship between average speed, distance and time (speed = distance ÷ time) The representation of a journey on a distance-time graph Relative motion: trains and cars passing one another Forces being needed to cause objects to stop or start moving, or to change their speed or direction of motion (qualitative only) Change depending on direction of force and its size. |
Forces as pushes or pulls, arising from the interaction between two objects Using force arrows in diagrams, adding forces in one dimension, balanced and unbalanced forces Forces measured in newtons, measurements of stretch or compression as force is changed Force-extension linear relation; Hooke’s Law as a special case Work done and energy changes on deformation Non-contact forces: gravity forces acting at a distance on Earth and in space, forces between magnets and forces due to static electricity. |
Waves on water as undulations which travel through water with transverse motion; these waves can be reflected, and add or cancel – superposition. Sound produced by vibrations of objects, in loud speakers, detected by their effects on microphone diaphragm and the ear drum; sound waves are longitudinal. Sound needs a medium to travel, the speed of sound in air, in water, in solids Pressure waves transferring energy; use for cleaning and physiotherapy by ultra-sound; waves transferring information for conversion to electrical signals by microphone. The similarities and differences between light waves and waves in matter Light waves travelling through a vacuum; speed of light Colours and the different frequencies of light, white light and prisms (qualitative only); differential colour effects in absorption and diffuse reflection. |
| New Skills: | Make measurements of distance and time and then calculate speeds of objects.
Use ratios and proportional reasoning to convert units and to compute rates. Recall and apply the equation for an object moving at constant speed the distance travelled in a specific time. Calculate the average acceleration of an object. |
Describe the interaction between pairs of objects which produce a force on each object. Calculate the weight of an object. Describe examples of the forces acting on an isolated object or system Use free body diagrams to describe qualitatively examples where several forces lead to a resultant force on an object, including balanced forces when the resultant force is zero. Give examples of the forces involved in stretching, bending or compressing an object Explain why, to change the shape of an object (by stretching, bending or compressing), more than one force has to be applied – this is limited to stationary objects only Describe the difference between elastic deformation and inelastic deformation caused by stretching forces. |
Describe wave motion in terms of their amplitude, wavelength, frequency and period. Apply the equation to calculate the frequency of a wave is the number of waves passing a point each second. Apply the equation to work out the speed of a wave. Identify amplitude and wavelength from given diagrams Describe a method to measure the speed of sound waves in air. Describe a method to measure the speed of ripples on a water surface. Give examples that illustrate the transfer of energy by electromagnetic waves. Give brief explanations why each type of electromagnetic wave is suitable for the practical application. |
| Links to the School Curriculum: |
Geography – Interpreting graphs Maths – Calculations Maths – Manipulating equations Maths – Graph skills and analysis PE – Speed + velocity – types of take-off for triple jump, high jump and long jump PE – Momentum+ throwing |
History, Geography, Psychology - Interpreting observations and other data, including identifying patterns and trends, making inferences and drawing conclusions presenting reasoned explanations, including relating data to hypotheses Ethics - being objective Maths - evaluating data in terms of accuracy, precision, repeatability and reproducibility and identifying potential sources of random and systematic error |
D&T - Carrying out experiments appropriately, having due regard to the correct manipulation of apparatus, the accuracy of measurements and health and safety considerations Maths – Applying and manipulating equations English – Invisible man, tricks of light Geography – Rivers |
| Enrichment Activities: |
Draw velocity–time graphs from measurements and interpret lines and slopes to determine acceleration Interpret enclosed areas in velocity–time graphs to determine distance travelled (or displacement)
Measure, when appropriate, the area under a velocity–time graph by counting squares.
Investigate the effect of varying the force on the acceleration of an object of constant mass, and the effect of varying the mass of an object on the acceleration produced by a constant force. |
Investigate the relationship between force and extension for a spring. |
Make observations to identify the suitability of apparatus to measure the frequency, wavelength and speed of waves in a ripple tank and waves in a solid and take appropriate measurements. Investigate how the amount of infrared radiation absorbed or radiated by a surface depends on the nature of that surface. |
| Web Links: | https://www.bbc.co.uk/bitesize/topics/ztmttv4https://www.freesciencelessons.co.uk/gcse-physics-paper-2/forces/ | https://www.bbc.co.uk/bitesize/topics/ztmttv4https://www.freesciencelessons.co.uk/gcse-physics-paper-2/forces/ | https://www.bbc.co.uk/bitesize/topics/z2j22nbhttps://www.freesciencelessons.co.uk/gcse-physics-paper-2/waves/ |

